Exhibit 99.1
Rigel Announces Fourth Quarter and Year End 2010 Financial Results
South San Francisco, Calif. — March 1, 2011 — Rigel Pharmaceuticals, Inc. (Nasdaq:RIGL) today reported financial results for the fourth quarter and year ended December 31, 2010.
For the fourth quarter of 2010, Rigel reported a net loss of $17.2 million, or $0.33 per share, compared to a net loss of $25.1 million, or $0.48 per share, in the fourth quarter of 2009. Weighted average shares outstanding for the fourth quarter of 2010 and 2009 were 52.2 million and 51.8 million, respectively.
There was no contract revenue reported in the fourth quarter of 2010. Contract revenue in the fourth quarter of 2009 was $750,000, comprised of a milestone payment from Daiichi Sankyo for the designation of the first lead compound related to the ligase oncology collaboration.
Rigel reported total operating expenses of $19.7 million in the fourth quarter of 2010, compared to $25.9 million in the fourth quarter of 2009. The decrease in operating expenses was primarily due to the completion of the transfer of the fostamatinib open label extension study to AstraZeneca AB (AZ) in September 2010.
In the fourth quarter of 2010, Rigel reported other income of approximately $2.4 million related to the Therapeutic Discovery Tax Credit Program (Section 48D of the Internal Revenue Code). Rigel received cash proceeds of $2.1 million in the fourth quarter of 2010 and will receive the remaining $0.3 million in 2011.
For the twelve months ended December 31, 2010, Rigel reported contract revenue of $125.0 million and net income of $37.9 million, or $0.73 and $0.72 per basic and diluted share, respectively, compared to contract revenue of $750,000 and a net loss of $111.5 million, or $2.73 per basic and diluted share, for the same period of 2009. The increase in contract revenue was due mainly to the $100.0 million upfront payment received pursuant to the exclusive worldwide license agreement with AZ for fostamatinib, that was fully recognized as revenue in 2010, as well as $25.0 million in milestone payments earned from AZ for the initiation of the phase 3 clinical trial program with fostamatinib in patients with RA and the completion of the transfer of the fostamatinib open label extension study to AZ.
As of December 31, 2010, Rigel had cash, cash equivalents and available for sale securities of $177.3 million, compared to $133.3 million as of December 31, 2009. Rigel expects to end 2011 with approximately $105.0 million in cash, cash equivalents and available for sale securities, which is expected to be sufficient to fund operations into 2013.
“2010 was a significant growth year for Rigel due to our successful collaboration with AstraZeneca and their launch of the OKSIRA global phase 3 clinical trial program with fostamatinib,” said James M. Gower, chairman and chief executive officer of Rigel. “We are
now concentrating on furthering the development of our next clinical projects, that include a novel oral JAK3 inhibitor intended for the treatment of transplant rejection and a different JAK3 inhibitor in an ophthalmic formulation intended for the treatment of Sjögren’s autoimmune-related dry eye,” he added. “We expect both of these programs to enter phase 1 clinical studies in late 2011.”
Pipeline/Program Update
As of March 2011, Rigel has seven programs in its development pipeline that are in varying stages of clinical or preclinical studies. The two most advanced programs are licensed to pharmaceutical partners: AZ has initiated the OSKIRA phase 3 clinical trial program with fostamatinib in patients with RA; and R343 (an inhaled syk inhibitor for chronic asthma) is currently in phase 1 with Pfizer. AZ has announced that they are on target to file a new drug application with the U.S. Food and Drug Administration in 2013 for fostamatinib in RA. Pfizer has informed Rigel that it plans on conducting an additional allergen challenge trial in 2011.
Rigel’s research and development teams are currently concentrating on the following five potential new products in the pipeline:
· JAK3 inhibitor (oral) - Rigel’s oral Janus kinase 3 (JAK3) inhibitor may provide a significant solution to the problem of organ transplant rejection for the approximately 90,000 people undergoing major organ transplants each year. Rigel expects to begin Phase 1 clinical trials with this program by the end of 2011.
· JAK3 inhibitor (ophthalmic) - Approximately 4 million people in the U.S. have Sjögren’s disease, an autoimmune disorder that affects the lacrimal glands of the eye. Present therapies for this chronic and painful condition are only mildly effective. Rigel has developed a soluble JAK3 inhibitor for topical ophthalmic use (eye drops) that it expects to enter into phase 1 clinical trials in 2011.
· PKC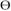 inhibitor- Protein Kinase C Theta (PKC
inhibitor- Protein Kinase C Theta (PKC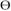 ) is an immune system activator whose expression is limited to certain immune and muscle cells, and has been shown to be significant in the progression of multiple sclerosis (MS). Rigel’s research is focused on developing a PKC
) is an immune system activator whose expression is limited to certain immune and muscle cells, and has been shown to be significant in the progression of multiple sclerosis (MS). Rigel’s research is focused on developing a PKC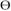 inhibitor that would target the inflammation of the nerve cells, characteristic of MS, thereby reducing the unpredictable and potentially devastating effects of the disease. Rigel expects to select an oral PKC
inhibitor that would target the inflammation of the nerve cells, characteristic of MS, thereby reducing the unpredictable and potentially devastating effects of the disease. Rigel expects to select an oral PKC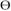 inhibitor product candidate in 2011.
inhibitor product candidate in 2011.
· ACVR2B inhibitor (Muscle Activation) — Patients with many serious diseases including diabetes, HIV, cancer and chronic heart failure are prone to develop cachexia, a disorder characterized by muscle atrophy. The loss of muscle critical to breathing and homeostasis is frequently the cause of death in patients with these serious diseases. In preclinical studies, Rigel’s oral ACVR2B inhibitors have been shown to block the cycle of muscle degradation. Rigel expects to begin clinical trials with this program, for the potential indication of ventilator associated disuse atrophy (VADA), in late 2012 or 2013.
· AMPK Activator (Muscle Endurance) - Another aspect of Rigel’s muscle metabolism research is focused on activating AMPK, an enzyme that regulates muscle energy and endurance. As an oral therapy this product candidate may be useful in aiding patients with diseases such as chronic obstructive pulmonary disease (COPD) or chronic heart failure (CHF) and others to regain the physical strength required to sustain everyday life. Rigel expects to begin clinical trials with this program in late 2012 or 2013.
About Rigel (www.rigel.com)
Rigel is a clinical-stage drug development company that discovers and develops novel, small-molecule drugs for the treatment of inflammatory and autoimmune diseases, as well as muscle disorders. Rigel’s pioneering research focuses on intracellular signaling pathways and related targets that are critical to disease mechanisms. Rigel’s productivity has resulted in strategic collaborations with large pharmaceutical partners to develop and market its product candidates. Current product development programs include fostamatinib, an oral syk inhibitor that has started its phase 3 clinical trial program for rheumatoid arthritis, and R343, an inhaled syk inhibitor that is in clinical trials for asthma.
This press release contains “forward-looking” statements, including, without limitation, statements related to Rigel’s expectations as to its year-end cash position and the sufficiency of its cash, cash equivalents and available for sale securities; identification of a novel programs for clinical development, the potential indications for treatment, and the timing of clinical trials; and the timing of filing a new drug application with respect to fostamatinib. Any statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “may,” “intend,” “believe,”“expect,” “potential,” and similar expressions are intended to identify these forward-looking statements. These forward-looking statements are based upon Rigel’s current expectations and involve risks and uncertainties. There are a number of important factors that could cause Rigel’s results to differ materially from those indicated by these forward-looking statements, including, without limitation, risks associated with Rigel’s need for additional capital, the timing and success of preclinical studies and clinical trials and the potential problems that may arise in the research and development and approval process, as well as other risks detailed from time to time in Rigel’s SEC reports, including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2010. Rigel does not undertake any obligation to update forward-looking statements and expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein.
###
Contact: Ryan D. Maynard
Phone: 650.624.1284
Email: invrel@rigel.com
Media Contact: Susan C. Rogers, Alchemy Consulting, Inc.
Phone: 650.430.3777
Email: susan@alchemyemail.com
STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
|
|
|
Three Months Ended December 31, |
|
Twelve Months Ended December 31, |
|
|
|
|
2010 |
|
2009 |
|
2010 |
|
2009 |
|
|
|
|
(unaudited) |
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
Contract revenues |
|
$ |
— |
|
$ |
750 |
|
$ |
125,000 |
|
$ |
750 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Research and development (see Note A) |
|
13,758 |
|
20,175 |
|
64,392 |
|
90,743 |
|
|
General and administrative (see Note A) |
|
5,911 |
|
5,677 |
|
25,291 |
|
20,903 |
|
|
Restructuring charges (see Note A) |
|
— |
|
— |
|
— |
|
1,141 |
|
|
Total operating expenses |
|
19,669 |
|
25,852 |
|
89,683 |
|
112,787 |
|
|
Income (loss) from operations |
|
(19,669 |
) |
(25,102 |
) |
35,317 |
|
(112,037 |
) |
|
Other income |
|
2,361 |
|
— |
|
2,361 |
|
— |
|
|
Interest income, net |
|
69 |
|
9 |
|
212 |
|
397 |
|
|
Income (loss) before income taxes |
|
(17,239 |
) |
(25,093 |
) |
37,890 |
|
(111,640 |
) |
|
Income tax benefit |
|
— |
|
— |
|
— |
|
93 |
|
|
Net income (loss) |
|
$ |
(17,239 |
) |
$ |
(25,093 |
) |
$ |
37,890 |
|
$ |
(111,547 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.33 |
) |
$ |
(0.48 |
) |
$ |
0.73 |
|
$ |
(2.73 |
) |
|
Diluted |
|
$ |
(0.33 |
) |
$ |
(0.48 |
) |
$ |
0.72 |
|
$ |
(2.73 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares used in computing net income (loss) per share: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
52,152 |
|
51,828 |
|
52,055 |
|
40,876 |
|
|
Diluted |
|
52,152 |
|
51,828 |
|
52,573 |
|
40,876 |
|
Note A
|
Stock-based compensation expense included in: |
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
2,008 |
|
$ |
2,628 |
|
$ |
9,025 |
|
$ |
8,937 |
|
|
General and administrative |
|
1,793 |
|
1,153 |
|
7,411 |
|
4,379 |
|
|
Restructuring charges |
|
— |
|
— |
|
— |
|
122 |
|
|
|
|
$ |
3,801 |
|
$ |
3,781 |
|
$ |
16,436 |
|
$ |
13,438 |
|
SUMMARY BALANCE SHEET DATA
(in thousands)
|
|
|
December 31, |
|
December 31, |
|
|
|
|
|
|
|
|
2010 |
|
2009(1) |
|
|
|
|
|
|
|
|
(unaudited) |
|
|
|
|
|
|
|
|
Cash, cash equivalents and available for sale securities |
|
$ |
177,295 |
|
$ |
133,318 |
|
|
|
|
|
|
Total assets |
|
186,695 |
|
140,744 |
|
|
|
|
|
|
Stockholders’ equity |
|
166,131 |
|
109,867 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Derived from audited financial statements
![]() inhibitor- Protein Kinase C Theta (PKC
inhibitor- Protein Kinase C Theta (PKC![]() ) is an immune system activator whose expression is limited to certain immune and muscle cells, and has been shown to be significant in the progression of multiple sclerosis (MS). Rigel’s research is focused on developing a PKC
) is an immune system activator whose expression is limited to certain immune and muscle cells, and has been shown to be significant in the progression of multiple sclerosis (MS). Rigel’s research is focused on developing a PKC![]() inhibitor that would target the inflammation of the nerve cells, characteristic of MS, thereby reducing the unpredictable and potentially devastating effects of the disease. Rigel expects to select an oral PKC
inhibitor that would target the inflammation of the nerve cells, characteristic of MS, thereby reducing the unpredictable and potentially devastating effects of the disease. Rigel expects to select an oral PKC![]() inhibitor product candidate in 2011.
inhibitor product candidate in 2011.