Exhibit 99.1
[LOGO]

Exhibit 99.1
[LOGO]

Rigel Conference Call FIT Phase 3 Study in ITP August 30, 2016 5:00am PT / 8:00am ET
Agenda Safe Harbor Statement D. Vance Introduction and Overview R. Rodriguez FIT 1 Phase 3 Results A. Duliege FIT 1 Phase 3 Commentary J. Bussel Q&A
Safe Harbor Statement In the conference call accompanying these slides, Rigel management will be making some forward-looking statements, including statements relating to Rigel's plans for the future clinical development of fostamatinib and the timing of results thereof. Any statements contained in this call that are not statements of historical fact may be deemed to be forward-looking statements. Words such as "anticipates," "plans," "intends," "expects" and similar expressions are intended to identify these forward-looking statements. These forward-looking statements are based on Rigel's current expectations and involve risks and uncertainties. There are a number of important factors that could cause Rigel's results to differ materially from those indicated by these forward-looking statements, including risks associated with the timing and success of clinical trials and other risks detailed in Rigel's SEC reports, including its Quarterly Report on Form 10-Q for the quarter ended June 30, 2016. Rigel expressly disclaims any obligation or undertaking to update the forward-looking statements discussed in this call.
Introduction and Overview Raul Rodriguez President and Chief Executive Officer

Participants Rigel Senior Management: Raul Rodriguez – President and Chief Executive Officer Anne-Marie Duliege, MD – Executive Vice President and Chief Medical Officer Donald G. Payan, MD – Executive Vice President and Chief Scientific Officer Dolly Vance – Executive Vice President, Corporate Affairs and General Counsel Ryan Maynard – Executive Vice President and Chief Financial Officer Principal Investigator: James Bussel, MD – Professor of Pediatrics, Pediatrics in Obstetrics and Gynecology, and Pediatrics in Medicine at Weill Cornell Medical College

ITP Background Significant unmet need: Characterized by the destruction of platelets by the body’s own immune system Increased risk of severe bleeding events Can result in serious medical complications or even death Heterogeneous patient population, difficult to predict which of available therapies will work, potentially including splenectomy Approximately 50,000-60,000 adult primary ITP patients in the United States (Orphan disease) Attractive market dynamics: Niche market Focused and identifiable prescriber base Need for new agents Neunert C, et al. Blood. 2011;117:4190-4207. Provan D and Newland AC. Adv Ther. 2015;32:875-887. Provan D, et al. Blood. 2010;115:168-186.
Why Fostamatinib? Novel mechanism of action Oral inhibitor of SYK, a key player in platelet destruction in ITP May uniquely address the underlying autoimmune basis of ITP by impeding platelet destruction Based on Phase 2 study: timely, substantial, and enduring benefit Primary endpoint responders do so within weeks of initiating treatment Initial platelet counts <20K increase to >100K Two patients taking Fostamatinib for 7+ years maintain attractive platelet levels over an extended period of time Safety Large safety database, primarily in patients with auto-immune disease (>5000 patient-years) Fostamatinib in ITP Braselmann S, et al. J Pharmacol Exp Ther. 2006;319:998-1008. Podolanczuk A, et al. Blood. 2009;113:3154-3160.
FIT 1 Phase 3 Program: Study 047 Results Anne-Marie Duliege, MD Executive Vice President and Chief Medical Officer
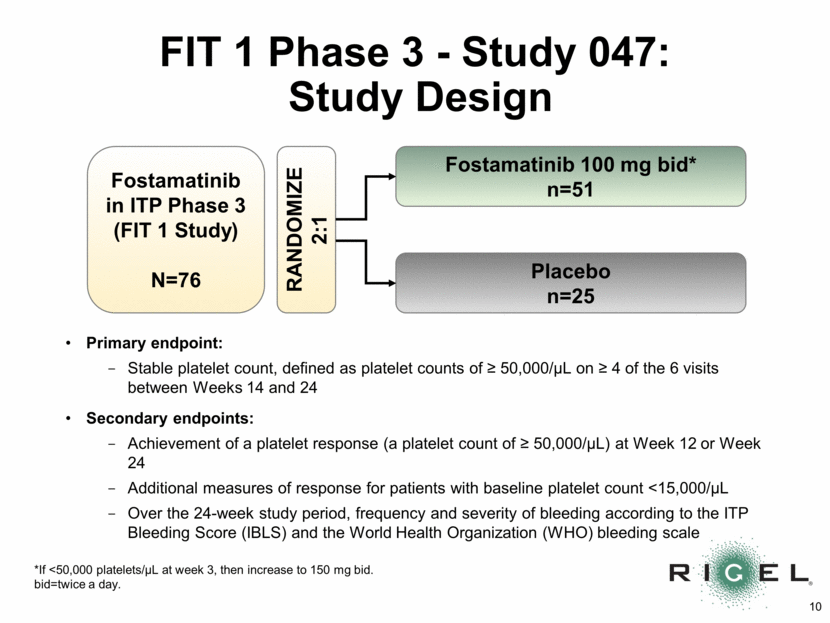
FIT 1 Phase 3 - Study 047: Study Design Fostamatinib in ITP Phase 3 (FIT 1 Study) N=76 Fostamatinib 100 mg bid* n=51 Placebo n=25 RANDOMIZE 2:1 Primary endpoint: Stable platelet count, defined as platelet counts of > 50,000/uL on > 4 of the 6 visits between Weeks 14 and 24 Secondary endpoints: Achievement of a platelet response (a platelet count of > 50,000/uL) at Week 12 or Week 24 Additional measures of response for patients with baseline platelet count <15,000/uL Over the 24-week study period, frequency and severity of bleeding according to the ITP Bleeding Score (IBLS) and the World Health Organization (WHO) bleeding scale *If <50,000 platelets/uL at week 3, then increase to 150 mg bid. bid=twice a day.
FIT 1 Phase 3 - Study 047: Relevant Patient Baseline Characteristics Patient Population: Adults with chronic/persistent ITP, defined as having consistently low platelet levels of <30,000 platelets/µL of blood All subjects have received prior treatment for ITP Fostamatinib N=51 Placebo N=25 Total N=76 Age, median (years) 57 57 57 Gender, n (%) Female Male 30 (59%) 21 (41%) 17 (68%) 8 (32%) 47 (62%) 29 (38%) Duration of ITP (years) Median Range 7.5 0.6 - 53 5.5 0.4 - 45 7 0.4 - 53 Prior treatments, n (%) Steroids Rituximab Thrombopoietic agents Splenectomy 46 (90%) 26 (51%) 26 (50%) 20 (39%) 25 (100%) 11 (44%) 15 (60%) 10 (40%) 71 (93%) 37 (49%) 41 (54%) 30 (39%) Median platelet count at Baseline 15,000 16,000 15,000
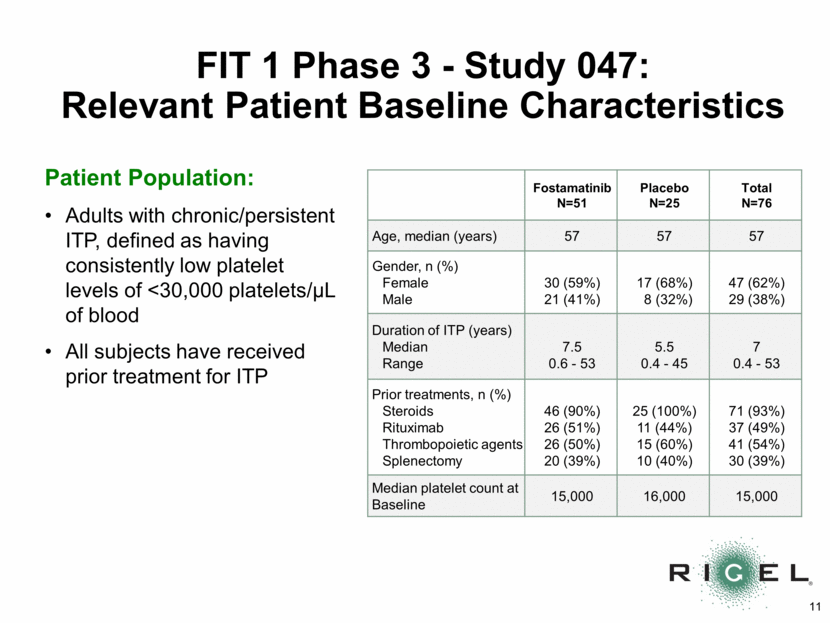
FIT 1 Phase 3 – Study 047: Primary Endpoint p=0.0261 n=9 *Stable platelet response (primary endpoint): platelet count of >50,000/uL on > 4 of the last 6 visits between Week 14 and Week 24 9/51=18% n=0 18% 0% 0% 20% 40% 60% 80% 100% Fostamatinib (n=51) Placebo (n=25) Percent of Patients Stable Platelet Response* by Week 24
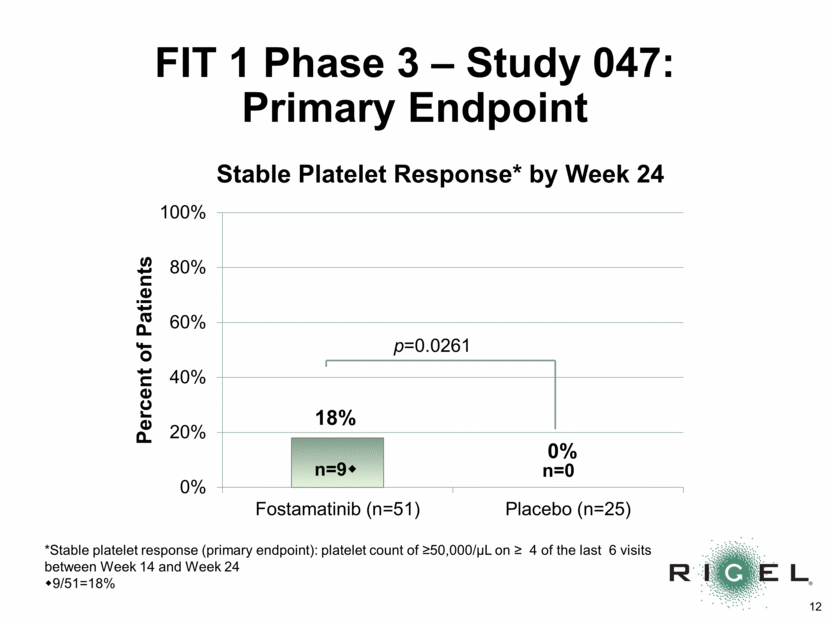
FIT 1 Phase 3 - Study 047: Adverse Events Number (n) and % of Patients with > one Adverse Event (AE) Fostamatinib N=51 Placebo N=25 n (%) N (%) Any AE* 49 (96%) 19 (76%) Serious AEs 8 (16%) 5 (20%) Treatment-related AEs 39 (77%) 7 (28%) Gastrointestinal complaints** 31 (61%) 5 (20%) Nausea 15 (29%) 1 (4%) Diarrhea 23 (45%) 4 (16%) Infection 17 (33%) 5 (20%) Hypertension during visit 18 (35%) 2 (8%) Transaminase elevation 11 (22%) 0 (0%) * AEs were generally mild (67%) or moderate (30%) ** Nausea, vomiting, diarrhea, or abdominal pain
FIT 1 Phase 3 – Study 047 Commentary James Bussel, MD Principal Investigator
Conclusions Key Findings: Met study primary endpoint of stable platelet response Safety profile consistent with prior experience. AEs related to GI were most frequent. AEs were generally mild or moderate. For Patients who met Primary Endpoint: Timely platelet response Substantial increase in platelet counts Platelet response was enduring Attractive opportunity for Rigel
Q&A
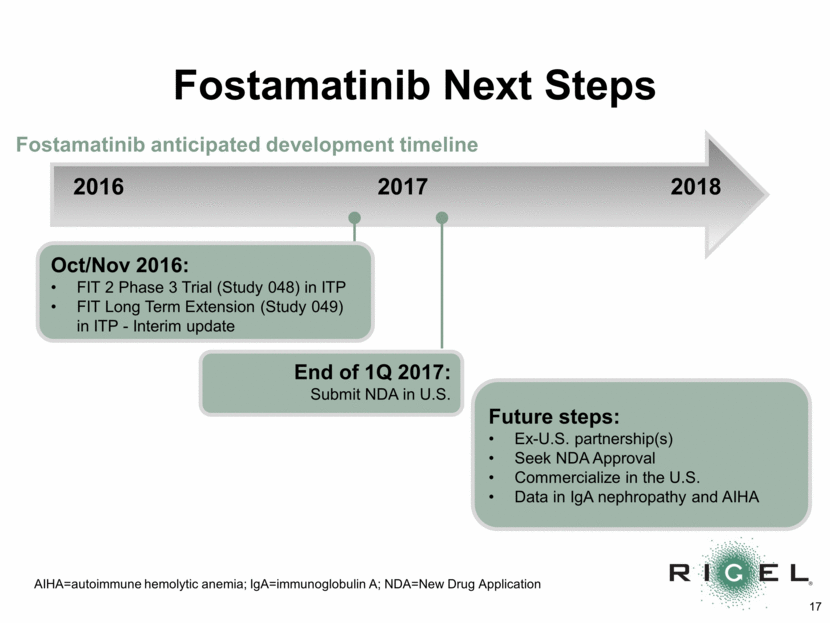
Fostamatinib Next Steps 2016 2017 2018 Future steps: Ex-U.S. partnership(s) Seek NDA Approval Commercialize in the U.S. Data in IgA nephropathy and AIHA Fostamatinib anticipated development timeline AIHA=autoimmune hemolytic anemia; IgA=immunoglobulin A; NDA=New Drug Application Oct/Nov 2016: FIT 2 Phase 3 Trial (Study 048) in ITP FIT Long Term Extension (Study 049) in ITP - Interim update End of 1Q 2017: Submit NDA in U.S.