Exhibit 10.2
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [**], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
SUPPLY AGREEMENT
This Supply Agreement (the “Supply Agreement”) is entered into as of September 3, 2024 (the “Effective Date”) by and between Rigel Pharmaceuticals, Inc., a Delaware company having an address at 611 Gateway Blvd., Suite 900, South San Francisco, CA 94080, USA (“Rigel”) and Kissei Pharmaceutical Co. Ltd., a Japanese company having an address at 19-48 Yoshino, Matsumoto, Nagano 399-8710, Japan (“Kissei”). Rigel and Kissei may be referred to herein individually as a “Party” or collectively as the “Parties”.
RECITALS
Whereas, Rigel, a biopharmaceutical company, has developed its proprietary compound olutasidenib, also known as REZLIDHIA® in the United States, which has been approved by the FDA for the treatment of adults with acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation when the disease has come back (relapsed) or has not improved after one or more treatments (refractory) **;
Whereas, Rigel and Kissei are parties to a certain Collaboration and License Agreement of even date hereof (the “Collaboration and License Agreement”), under which Rigel has granted Kissei the right to develop and commercialize olutasidenib in the Kissei Territory; and
Whereas, the Collaboration and License Agreement contemplates that Rigel will manufacture, or have manufactured, and supply olutasidenib to Kissei for development and commercial use, and Rigel is willing to manufacture and supply olutasidenib to Kissei, on the terms and conditions set forth below.
Now, Therefore, in consideration of the foregoing premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
Article 1
DEFINITIONS
Capitalized terms used in this Supply Agreement but not defined herein shall have the meanings set forth in the Collaboration and License Agreement.
1.1“Batch” means the quantity of a Product produced in a single production run of such Product. 1.2“Business Day” means a day that is not a Saturday, Sunday, or a day on which banking institutions in ** are authorized by Applicable Law to remain closed. 1.3“Claim” had the meaning set forth in Section 9.3.
1.4“Collaboration and License Agreement” has the meaning set forth in the Recitals.
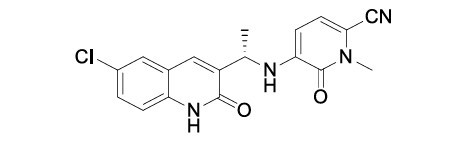
1.5“Compound” means olutasidenib, having the chemical structure set forth in Exhibit A.
1.6 “Drum” means the quantity of Product, which is a portion of a Batch, supplied by Rigel to Kissei as mutually agreed upon in the Specification.